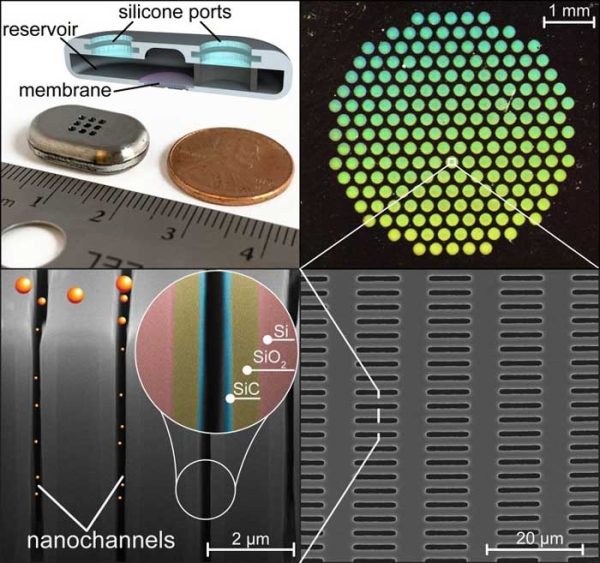
Nanofluidic implants for treatment and prevention of chronic diseases (nDS)
The nanochannel drug delivery system (nDS) is a long-acting subcutaneous implant for continuous, sustained release of therapeutics to treat or prevent chronic diseases. At the core of the nDS technology is an implantable silicon nanofluidic membrane, within which the nanochannel size, numbers and surface properties can be tuned to achieve the desired release rate of any therapeutics.
The nDS maintains drug levels within the desired therapeutic window without fluctuations for maximal efficacy and minimal adverse effects. Drug release duration can be extended via minimally invasive transcutaneous refilling through the silicone ports. The nDS is validated across a variety of clinical indications in small and large preclinical disease-relevant models. Our priority therapeutic area includes HIV pre-exposure prophylaxis (PrEP), where improved medication adherence is fundamental to achieving epidemic control.
Reference: Pons-Faudoa, F.P. er al. Science Translational Medicine 2023
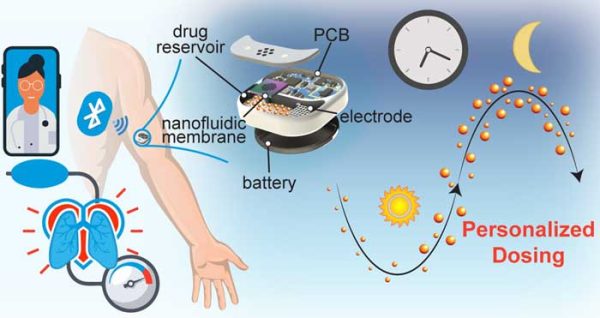
Remotely controlled tunable drug delivery devices
Our remotely controlled implant allows for tunable drug delivery via FDA-approved low–energy Bluetooth through an app on a mobile device or computer. The implant adopts buried polySilicon electrodes within a silicon nanochannel membrane to control drug release via low-voltage electric potential. The generated electric field creates an ionic redistribution inside the channels that electrostatically gates the transport of drug molecules. We are currently investigating remote controlled delivery in preclinical models for chronotherapeutic or telemedicine applications. Our innovation could advance the field of personalized medicine, where dosing is programmed according to individual need.
Reference: Di Trani, N.; et al. Lab on a Chip 2020.
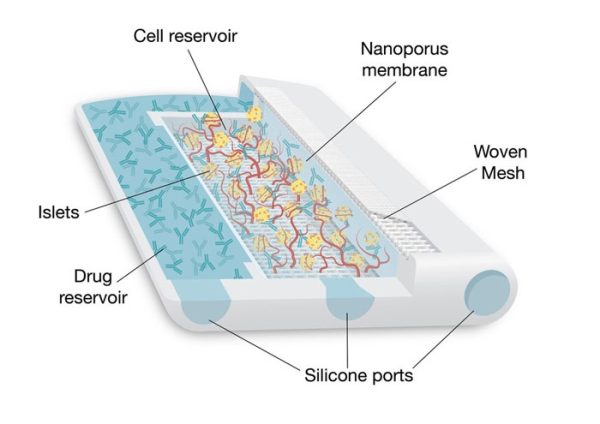
3D printed cell encapsulation device for endocrine cell transplantation (NICHE)
The Neovascularized Implantable Cell Homing and Encapsulation (NICHE) platform is a 3D printed polymeric implant for endocrine cell transplantation. The key innovation of the NICHE is the integration of local delivery of immunosuppressant drugs to a fully vascularized transplant microenvironment within the same implant. This unique design limits immunosuppressant delivery to the transplant site to prevent immune rejection of transplanted cells, while the vascularized microenvironment supports long-term engraftment. As such, transplant recipients are spared from the toxic side effects of whole-body immunosuppression. Loading and refilling of drugs or cells are achieved through silicon ports via a simple injection procedure. We have investigated the NICHE in small and large preclinical models, as a step toward clinical translation. Our current therapeutic focus includes Type 1 Diabetes management using pancreatic beta islets.
Reference: Paez-Mayorga, J. et al. Nature Communication 2022.
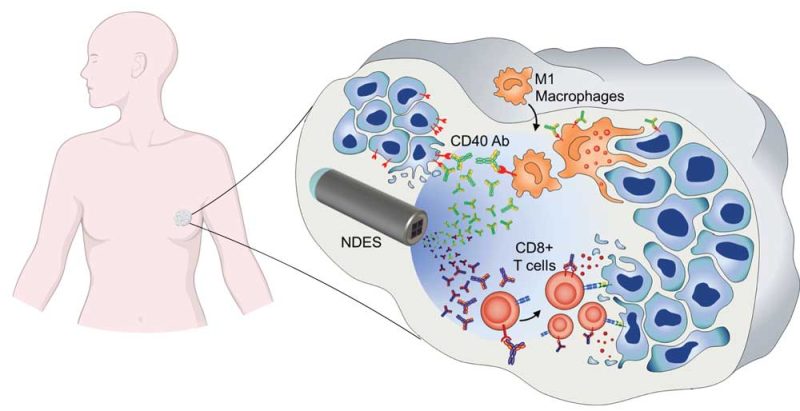
Nanofluidic Drug-Eluting Seed for intratumoral drug delivery (NDES)
The nanofluidic drug-eluting seed (NDES) is a tiny intratumoral implant that uses a silicon nanofluidic membrane for sustained local drug delivery. By delivering therapeutics directly to the tumor, the NDES could improve treatment effectiveness and avoid exposing the body to harmful drug side effects. The NDES is drug- and tumor-agnostic and can be adopted as a drug-eluting radiopaque fiducial or for image-guided theranostic approaches. We have investigated local delivery of chemotherapy, immunotherapy, and imaging contrast agents in various murine cancer models. Current therapeutic focus entails intratumoral immunotherapy, based on the premise that local priming of the anti-tumor immune response can yield durable systemic immunity with minimal toxicity. Combination studies of intratumoral immunotherapy with radiotherapy are currently ongoing in various murine cancer models, including breast cancer and pancreatic cancer. Our innovation aims to change cancer treatment paradigm in a safer and more effective manner.
Reference: Liu, H.-C. et al., Advanced Science 2023
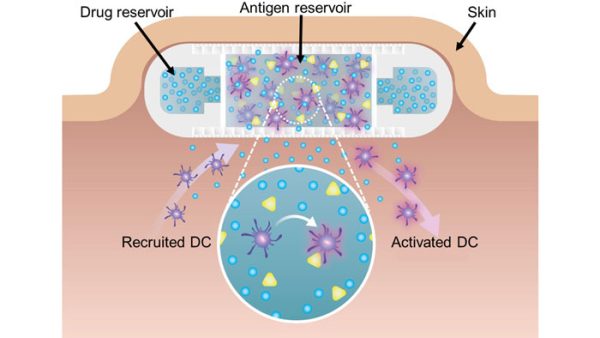
In situ immunomodulation for neoadjuvants (NanoLymph)
The NanoLymph is a 3D printed neoadjuvant delivery platform that acts as an immunostimulatory niche to generate antigen-specific immune responses through the continuous release of immunomodulatory agents. The NanoLymph has two reservoirs to house immunostimulatory drugs and antigens, respectively. Continuous drug release stimulates dendritic cells (DC) recruitment and immune activation against relevant antigens with the goal of achieving durable memory responses. Current studies focus on prophylactic and therapeutic delivery of cancer neoadjuvants in murine triple negative breast cancer and melanoma. Due to flexibility in immunomodulatory agents and antigens, the NanoLymph can be used for other clinical indications including allergy immunomodulation.
Reference: Viswanath, D. I. et al. Biomaterials. 2022.

Microgravity research on the International Space Station
The Center for Space Nanomedicine was founded to develop nanotechnology for biomedical use on-Earth and in space. Studies investigated on the International Space Station (ISS) National Laboratory aim to develop telemedicine innovations and examine new theories on nanoscale diffusion in microgravity environments and under orbital velocity movement. By working with CASIS and NASA and executing experiments in this unique environment, we have expanded the scope of scientific investigation at nanoscale levels. We are currently working on different projects in collaboration with the ISS National Laboratory, including:
- Investigation of the effect of outer space on carbon fiber reinforced polymers provided by Automobili Lamborghini (Learn more)
- Testing of the remotely controlled nanofluidic implant on the ISS (Learn more)
- Testing of sustained delivery implant for an anti-hypertrophy drug in a murine model aboard the ISS, in collaboration with Novartis (Learn more)
- Investigation of microfluidics transport physics in microgravity (Learn more)
Research
Nanofluidic implants for treatment and prevention of chronic diseases (nDS)

The nanochannel drug delivery system (nDS) is a long-acting subcutaneous implant for continuous, sustained release of therapeutics to treat or prevent chronic diseases. At the core of the nDS technology is an implantable silicon nanofluidic membrane, within which the nanochannel size, numbers and surface properties can be tuned to achieve the desired release rate of any therapeutics.
The nDS maintains drug levels within the desired therapeutic window without fluctuations for maximal efficacy and minimal adverse effects. Drug release duration can be extended via minimally invasive transcutaneous refilling through the silicone ports. The nDS is validated across a variety of clinical indications in small and large preclinical disease-relevant models. Our priority therapeutic area includes HIV pre-exposure prophylaxis (PrEP), where improved medication adherence is fundamental to achieving epidemic control.
Reference: Pons-Faudoa, F.P. er al. Science Translational Medicine 2023
Remotely controlled tunable drug delivery devices

Our remotely controlled implant allows for tunable drug delivery via FDA-approved low–energy Bluetooth through an app on a mobile device or computer. The implant adopts buried polySilicon electrodes within a silicon nanochannel membrane to control drug release via low-voltage electric potential. The generated electric field creates an ionic redistribution inside the channels that electrostatically gates the transport of drug molecules. We are currently investigating remote controlled delivery in preclinical models for chronotherapeutic or telemedicine applications. Our innovation could advance the field of personalized medicine, where dosing is programmed according to individual need.
Reference: Di Trani, N.; et al. Lab on a Chip 2020.
3D printed cell encapsulation device for endocrine cell transplantation (NICHE)

The Neovascularized Implantable Cell Homing and Encapsulation (NICHE) platform is a 3D printed polymeric implant for endocrine cell transplantation. The key innovation of the NICHE is the integration of local delivery of immunosuppressant drugs to a fully vascularized transplant microenvironment within the same implant. This unique design limits immunosuppressant delivery to the transplant site to prevent immune rejection of transplanted cells, while the vascularized microenvironment supports long-term engraftment. As such, transplant recipients are spared from the toxic side effects of whole-body immunosuppression. Loading and refilling of drugs or cells are achieved through silicon ports via a simple injection procedure. We have investigated the NICHE in small and large preclinical models, as a step toward clinical translation. Our current therapeutic focus includes Type 1 Diabetes management using pancreatic beta islets.
Reference: Paez-Mayorga, J. et al. Nature Communication 2022.
Nanofluidic Drug-Eluting Seed for intratumoral drug delivery (NDES)

The nanofluidic drug-eluting seed (NDES) is a tiny intratumoral implant that uses a silicon nanofluidic membrane for sustained local drug delivery. By delivering therapeutics directly to the tumor, the NDES could improve treatment effectiveness and avoid exposing the body to harmful drug side effects. The NDES is drug- and tumor-agnostic and can be adopted as a drug-eluting radiopaque fiducial or for image-guided theranostic approaches. We have investigated local delivery of chemotherapy, immunotherapy, and imaging contrast agents in various murine cancer models. Current therapeutic focus entails intratumoral immunotherapy, based on the premise that local priming of the anti-tumor immune response can yield durable systemic immunity with minimal toxicity. Combination studies of intratumoral immunotherapy with radiotherapy are currently ongoing in various murine cancer models, including breast and pancreatic cancer. Our innovation aims to change cancer treatment paradigm in a safer and more effective manner.
Reference: Liu, H.-C. et al., Advanced Science 2023
In situ immunomodulation for vaccinations (NanoLymph)

The NanoLymph is a 3D printed vaccine platform that acts as an immunostimulatory niche to generate antigen-specific immune responses through the continuous release of immunomodulatory agents. The NanoLymph has two reservoirs to house immunostimulatory drugs and antigens, respectively. Continuous drug release stimulates dendritic cells (DC) recruitment and immune activation against relevant antigens with the goal of achieving durable memory responses. Current studies focus on prophylactic and therapeutic cancer vaccination in murine triple negative breast cancer and melanoma. Due to flexibility in immunomodulatory agents and antigens, the NanoLymph can be used for other clinical indications including allergy immunomodulation.
Reference: Viswanath, D. I. et al. Biomaterials. 2022.
Microgravity research on the International Space Station

The Center for Space Nanomedicine was founded to develop nanotechnology for biomedical use on-Earth and in space. Studies investigated on the International Space Station (ISS) National Laboratory aim to develop telemedicine innovations and examine new theories on nanoscale diffusion in microgravity environments and under orbital velocity movement. By working with CASIS and NASA and executing experiments in this unique environment, we have expanded the scope of scientific investigation at nanoscale levels. We are currently working on different projects in collaboration with the ISS National Laboratory, including:
- Investigation of the effect of outer space on carbon fiber reinforced polymers provided by Automobili Lamborghini (Learn more)
- Testing of the remotely controlled nanofluidic implant on the ISS (Learn more)
- Testing of sustained delivery implant for an anti-hypertrophy drug in a murine model aboard the ISS, in collaboration with Novartis (Learn more)
- Investigation of microfluidics transport physics in microgravity (Learn more)
